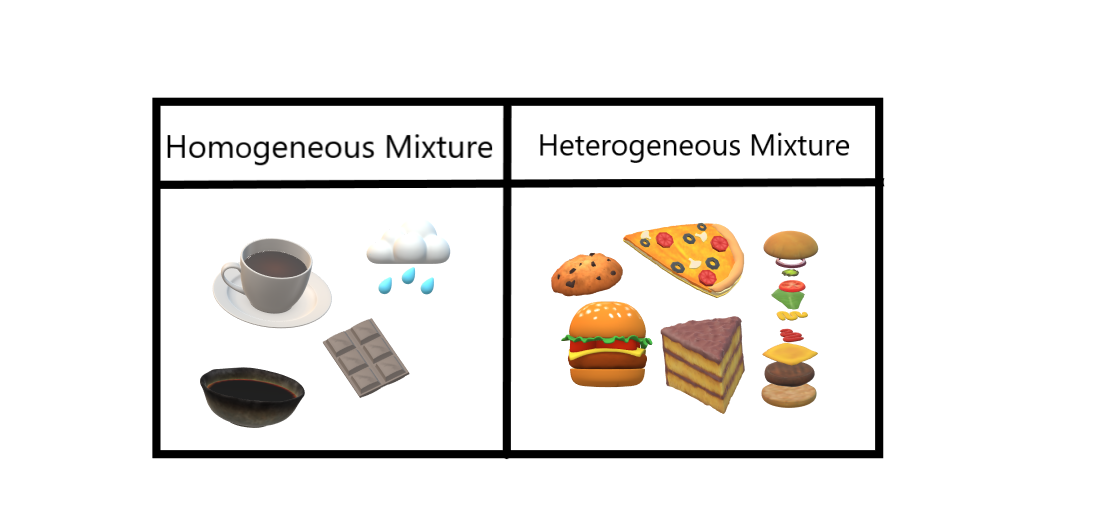
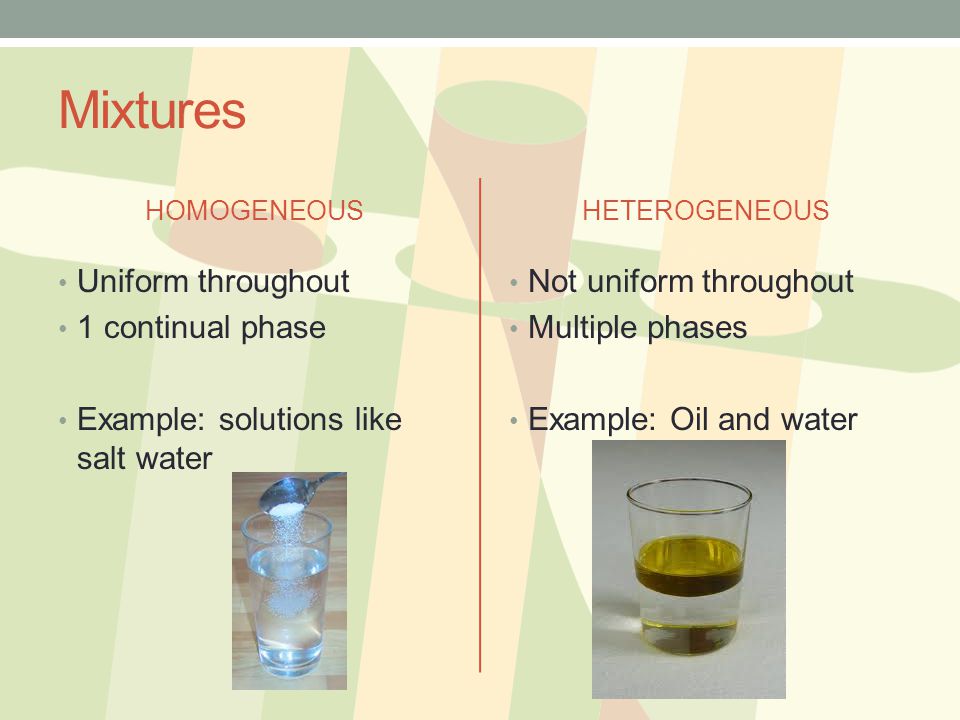
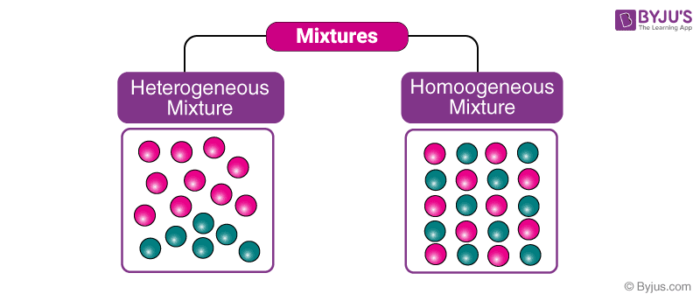
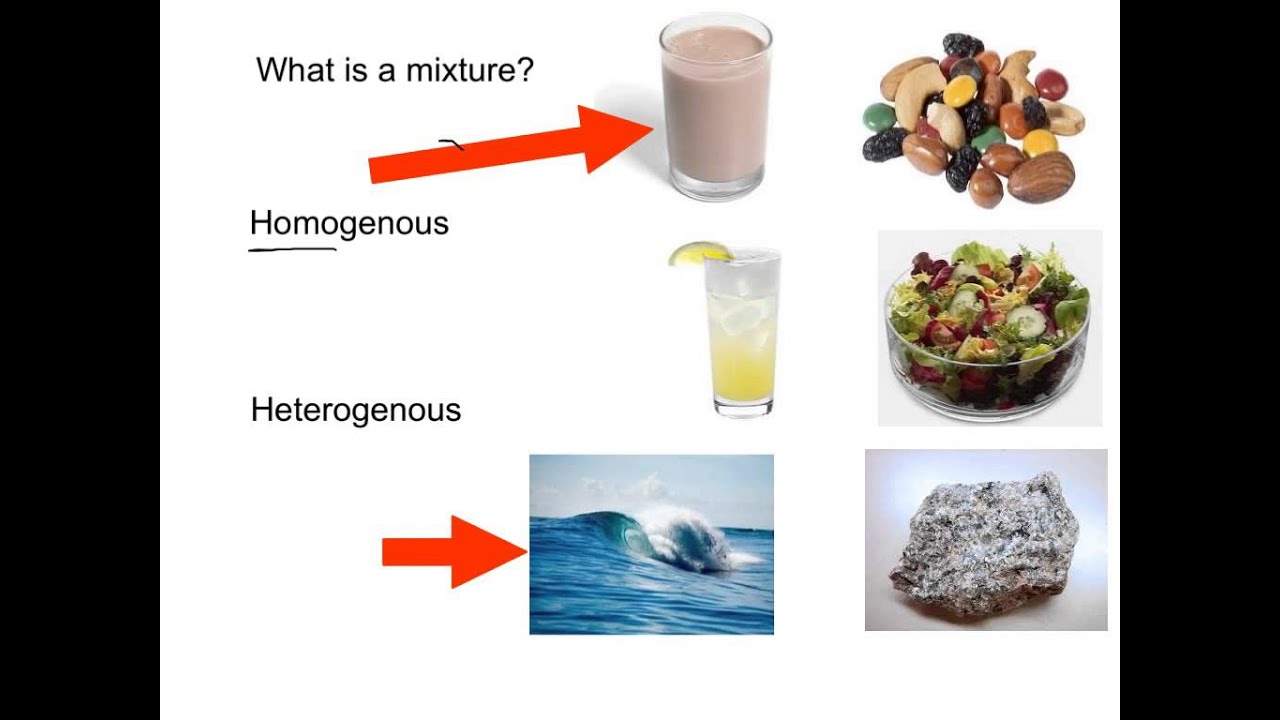
A soft drink is an example of a homogeneous mixture In soft drinks, the sweetener, carbon dioxide bubbles, water and additional ingredients are uniformly distributed However, a soft drink with ice is a heterogeneous mixture The ice andFor example Homogeneous mixtures include singlephase substances (the same state of matter), such as coffee with creamer (both liquid) or sterling silver (made with silver and copper) Heterogeneous mixtures include multiphase substances (different states of matter), such as sand and water (solid and liquid) or carbonated drinks (gas and liquid)Homogeneous mixture Heterogeneous mixture 1) These are called as solutions These are called as suspensions/colloids 2) Substances are Uniformly distributed These substances are Unevenly distributed 3) These are not visible to the naked eye, but visible through the microscope
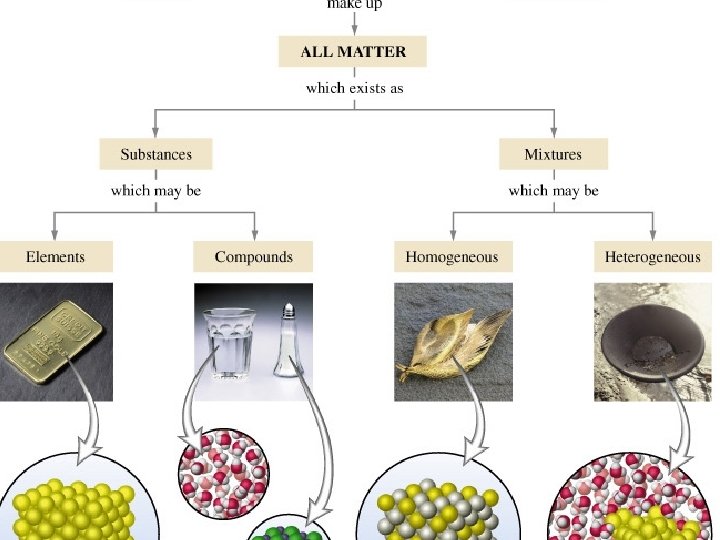
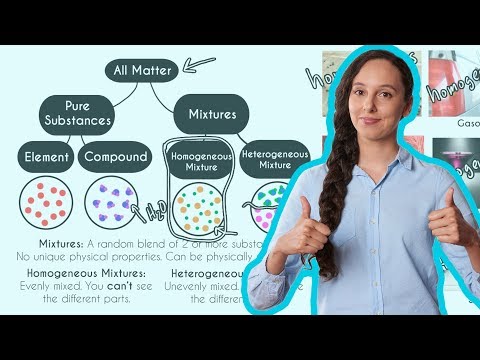
Classes Of Matter Elements Compounds And Mixtures Matter
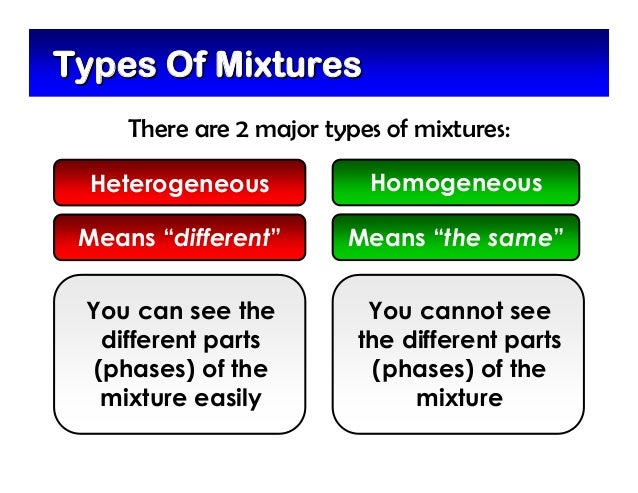
Heterogeneous and homogeneous examples
Heterogeneous and homogeneous examples- As an example, 2SO 2(g) O 2(g) ⇌ 2SO 3(g) is a homogeneous equilibrium and O 2(g) 2C (s) ⇌ 2CO (g) is an example for a heterogeneous equilibrium Below infographic tabulates the difference between homogeneous and heterogeneous equilibrium Summary – Homogeneous vs Heterogeneous EquilibriumHeterogeneous mixture is a mixture with a nonuniform composition When you mix two components that remain separate from each other, that mixture is called a Heterogeneous mixture Concrete is an example of a Heterogenous mixture A mixture of Cement and Water A mixture of cold drinks and ice cube is also an example of a Heterogeneous mixture




Examples Of Homogeneous Mixtures And Heterogenous Mixtures Chemistry Youtube
In this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference between There are several examples of homogeneous mixtures encountered in everyday life Air Sugar water Rainwater Vodka Vinegar Dishwashing detergent SteelTypical examples involve a solidcatalyst with the reactants as either liquids or gases Note It is important that you remember the difference between the two terms heterogeneousand homogeneous heteroimplies different(as in heterosexual) Heterogeneous catalysis has the catalyst in a different phase from the reactants
In homogeneous equilibrium, all substances are in the same phase In heterogeneous equilibrium, substances are in different phases Key Terms equilibrium The state of a reaction in which the rates of the forward and reverse reactions are the same heterogeneous solution A solution composed of different states of matter Homogeneous Vs Heterogeneous Teams The makeup of a team, including the mix of cultures and personalities, can make or break its effective output and viability A homogeneous team would include people who are as similar as possible, with similar points of view, learning abilities and life experiences Heterogeneous teams include a mixture of From the practical point of view, homogeneous welding is always easier compared to heterogeneous welding due to inherent chemical compatibility among filler and base metals Various similarities and differences between homogeneous welding and heterogeneous welding are discussed in the following sections
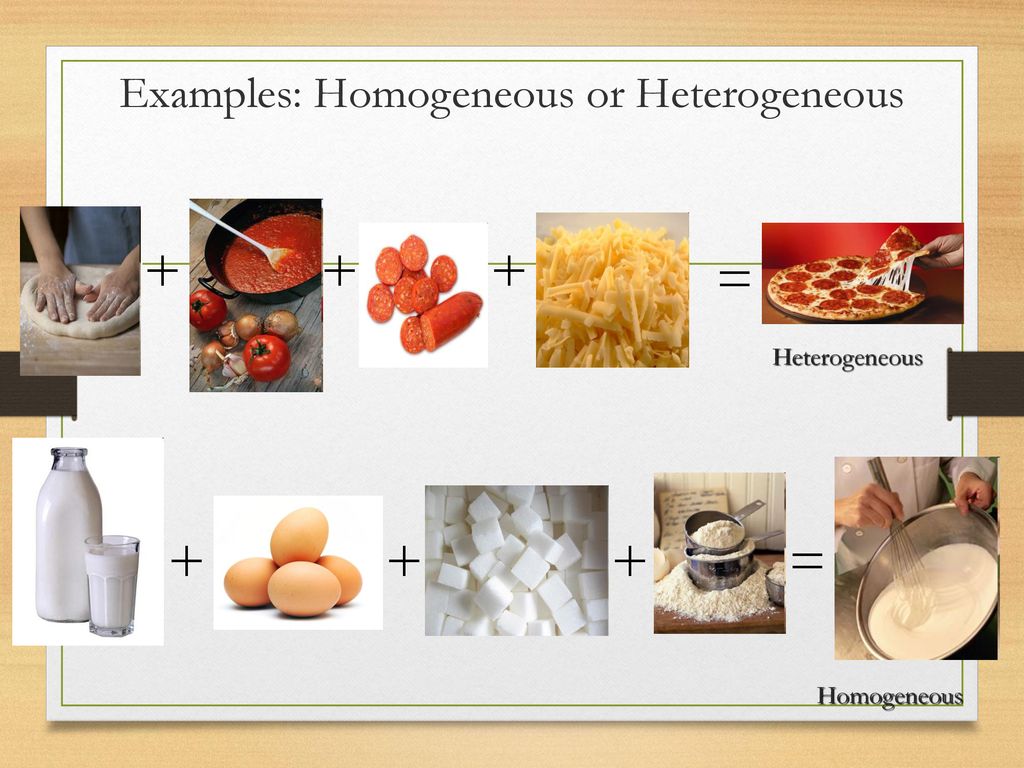
Start studying Heterogeneous vs Homogeneous Mixtures Learn vocabulary, terms, and more with flashcards, games, and other study toolsExamples of Homogeneous and Heterogeneous Mixtures Examples of heterogeneous mixtures would be ice cubes (before they melt) in soda, cereal in milk, various toppings on a pizza, toppings in frozen yogurt, a box of assorted nutsEven a mixture of oil and water is heterogeneous because the density of water and oil is different, which prevents uniform distribution in the mixture Entropy allows for heterogeneous substances to become homogeneous over time A heterogeneous mixture is a mixture of two or more compounds Examples are mixtures of sand and water or sand and iron filings, a conglomerate rock, water and oil, a




Heterogeneous And Homogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Middle School Science Resources




Fun Activity To Reinforce The Differences Between Heterogeneous And Homogeneous Mixture Heterogeneous Mixture Sorting Activities Teaching Middle School Science
The formations of black holes, volcano eruptions, and the popular DietCokeandMentos experiment are further examples involving a nucleation step *Fotos Kris Dux and Gaetan Lee under Creative Commons License Homogeneous and heterogeneous nucleation Heterogeneous nucleation begins on alien surfaces or particles, or preexisting nuclei in the Examples of solutions include things like mud, dust suspended in the air, granite, vinaigrette, and sand Homogenization The process of homogenization is what turns a heterogeneous mixture into a homogeneous mixture One of the most notable examples of homogenization is the process that produces homogenized milk Heterogeneous mixtures These are those in which the substances that make up the mixture (eg oil and water ) can be distinguished at first glance That is why it is said that they are not uniform since the substances do not combine The same goes for a salad of, for example, lettuce and tomato




3 Examples Of Heterogeneous And Homogeneous Boundary Layer Reactions Download Table




Separation Methods Types Of Solutions Mixtures Solution A Homogeneous Mixture Of Two Or More Compounds Where A Solute Has Dissolved In A Solvent Solution Ppt Download
Heterogeneous and Homogeneous Mixtures In the lesson, you learned about heterogeneous mixtures and homogeneous mixtures Another name for a homogeneous Examples include chocolate chip cookies, soda with ice, a sandwich, pizza, and tossed salad A heterogeneous mixture is defined as a mixture that has a nonuniform composition In other words, its composition varies from one location to another In contrast, a homogeneous mixture has a uniform composition Its appearance and composition are the Example Mixture of Salt and Iron Filings Note Heterogeneous and homogeneous mixture may be a Matter of Scale It means that a mixture may look homogeneous from a distance But if we look closely with help of a microscope, it may appear to be heterogeneous mixture (We may be able to identify individual component clearly)



Types Of Catalysis




Examples Of Homogeneous Mixtures And Heterogenous Mixtures Chemistry Youtube
Another typical example of heterogeneous equilibrium includes reaction of steam with red hot carbon Equation is given below – H2O(g) C(s) ↔ H2 (g) CO(g) As you can see above reaction is in equilibrium and water steam, carbon monoxide, hydrogen is present in gaseous phase while red hot carbon is in solid phaseStainless steel is made from a homogeneous mixture of iron, chromium, and nickel Air is a homogeneous mixture of nitrogen gas (78%), oxygen gas (21%), and small amounts of various other gases Oil, vinegar, dishwashing liquid, brass, wine, blood, seawater, natural gas, etc What is a heterogeneous mixture?Heterogeneous mixtures (mixtures where you can see the different parts) are distinctly different from homogeneous mixtures that create a uniform mixture To help to understand these two concepts, a heterogeneous mixture is like hot cocoa with marshmallows floating in it



Www Ahschools Us Cms Lib Mn Centricity Domain 4752 Matterpdf Pdf



Homogeneous And Heterogeneous Mixtures Geeksforgeeks
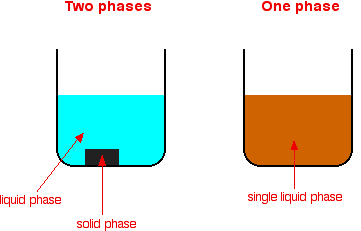
Examples of homogeneous mixtures include Salty water — a mixture of salt and water Ruby — a mixture of Al 2 O 3 and Cr 2 O 3 Gasoline — a mixture of various hydrocarbons Brass — a mixture of Cu and Zn Air without clouds — a mixture of various gasesHomogeneous vs Heterogeneous Networks Homogeneous networks are networks where all the nodes have the same function in the network One user is interchangeable with the next in the basic function they perform In a landline telephone network, for example, each node (telephone) performs basically the same function as any other, and people tendBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase




Research Mixtures For Kids




Examples Of Homogeneous Mixtures Solid Liquid And Gas
6 rows Through combining two or more substances, a mixture is produced A homogeneous solution tends to• Apple juice is homogeneous • Orange juice with pulp is heterogeneous • Chocolate dough is homogeneous • Italian salad dressing is heterogeneous Heterogeneous vs Homogeneous Assays After giving examples for practical examples of Biosensors, todays articles should classify differences between heterogeneous and homogeneous assaysTo properly understand the differences between both assays, a few definitions need to be clarified




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Scienceman Digital Lesson Mixtures Homogeneous Heterogeneous Youtube
Activity This one page worksheet will give your students practice with heterogeneous and homogeneous mixtures The topics of the questions include prefix meanings, examples, differences between the two types of mixtures, and the definition of mixtures The worksheet involves critical thinking skills and will Heterogeneous products are products with attributes that are significantly different from each other, which makes it difficult to substitute one product for another An example of a heterogeneous product is a computer Commodities are generally a good example of homogeneous products An example of a heterogeneous product is a computer You really can't substitute a PC for a Mac, because each computer platform is too different On




Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Youtube



Ppt Objective I Will Distinguish Between Homogeneous And Heterogeneous Mixtures Powerpoint Presentation Id
Examples Solutions are examples of the homogenous mixture Colloids and suspensions are examples of a heterogeneous mixture Homogeneous Mixture Examples There are several examples of homogeneous mixtures encountered in everyday life Air Sugar water Rain water Vodka Vinegar Dishwashing detergent Steel A heterogeneous mixture is a mixture where the components of the mixture are not uniform or have localized regions with different properties 3 Responses to "Homogeneous vs Heterogeneous" venqax on 444 pm "When lawmakers speak of the necessity to create "a homogeneous multiracial society," their goal is a society in which race, ethnicity, and religion are of secondary importance to a sense of civic equality and consciousness of a shared culture"



Is Salty Water Homogeneous Or Heterogeneous




Heterogeneous Images Stock Photos Vectors Shutterstock
The components in a heterogeneous mixture can easily be seen with a naked eye, but for better results, you can utilize a microscope Examples Homogeneous Mixture Some examples of homogeneous mixtures are most alloys, seawater, brass, vinegar, air, blood, natural gas, etc Heterogeneous Mixture Salt and pepper together make a heterogeneousAbout heterogeneous mixture homogeneous mixture worksheet The heterogeneous mixture – homogeneous mixture worksheet with answer key is below The worksheet gives common examples of mixtures, in addition to some pure, unmixed substances Learn how to classify these examples of mixtures belowIn this animated lecture, I will teach you about 10 examples of homogeneous mixtures and 10 examples of heterogeneous mixtures, the meaning of homogeneous, t




Which Of The Following Are Examples Of Hom Clutch Prep




Homogeneous And Heterogeneous Mixtures Worksheet 4 Youtube
Homogeneous catalysts work better in lowtemperature conditions (less than 250 0 C) In which the reactants and catalyst are in a similar phase Example 2SO 2 (g) O 2 (g) —– No(g) → 2SO 3 (g) Heterogeneous catalysis Heterogeneous catalysts are catalytic compounds that are in a contradictory phase from that of the phase of theHeterogeneous Mixture Homogeneous Mixture Worksheet Exercise 1 Describe the following as an element, a compound, a homogenous mixture, or a heterogeneous mixture a) a pure silver ring _____ b) pure water _____ c) sugar water _____ d) pure magnesium oxide (MgO) _____ e) For example, a salad Unlike homogeneous mixtures, in heterogeneous mixtures it is very easy to identify, even with the naked eye, what are the different components that make them up This makes it much easier to separate these mixes at the same time For example water and oil / water and sand




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com




Heterogeneous Homogeneous Mixture Card Sort For Matter In Chemistry



Mixture




Heterogeneous Homogeneous Mixture Card Sort For Matter In Chemistry Homogeneous Mixture Heterogeneous Mixture Sorting Cards




Heterogeneous And Homogeneous Mixtures Examples Pdf Homogeneity And Heterogeneity Mixture




Explain The Difference Between A Homogeneous And Heterogeneous Mixture Give An Example For Each Brainly Com
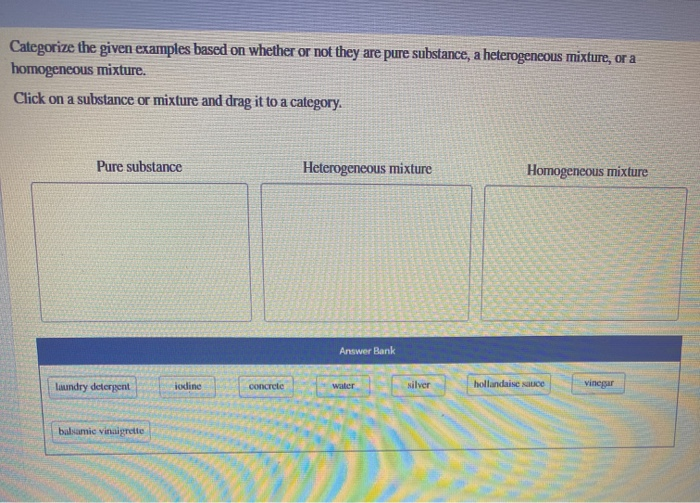



Categorize The Given Examples Based On Whether Or Not Chegg Com




Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet Teaching Chemistry Middle School Science Experiments Middle School Science Resources



Q Tbn And9gcsbe Ybqypf3mkoozl7w6krqjqo5iirs Nvzkdphtkiwz1wjxbm Usqp Cau



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture Venn Diagram Examples Venn Diagram Template




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



Heterogeneous And Homogeneous Mixture Differences Videos Examples




What Is A Homogeneous Mixture Definition And Examples




What Is The Difference Between A Homogeneous Mixture And A Heterogeneous Mixture Bitwise Academy



Separation Methods Ppt Download




Pure Substances And Mixtures Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture



Homogeneous And Heterogeneous Mixtures




Q1 Define Homogenous And Heterogeneous Mixture And Chegg Com
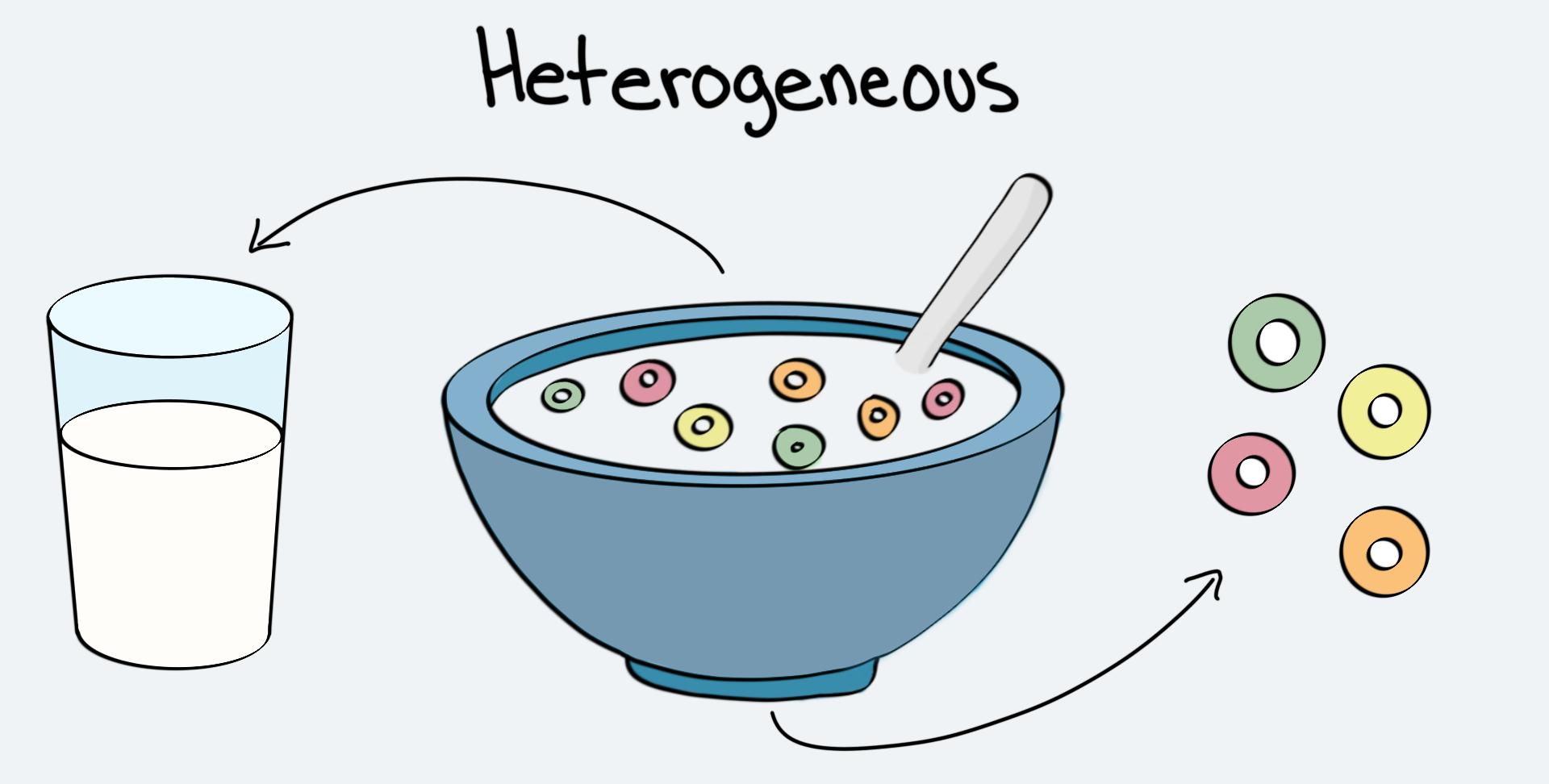



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Matter Worksheets
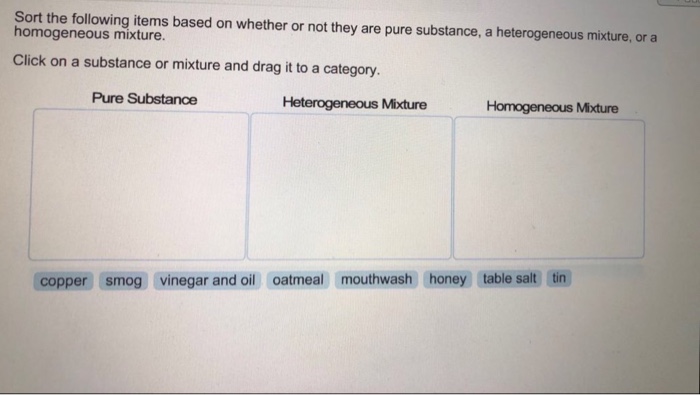



Sort The Following Items Based On Whether Or Not They Chegg Com




5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends




Solutions And Mixtures Flashcards Quizlet



Mixture
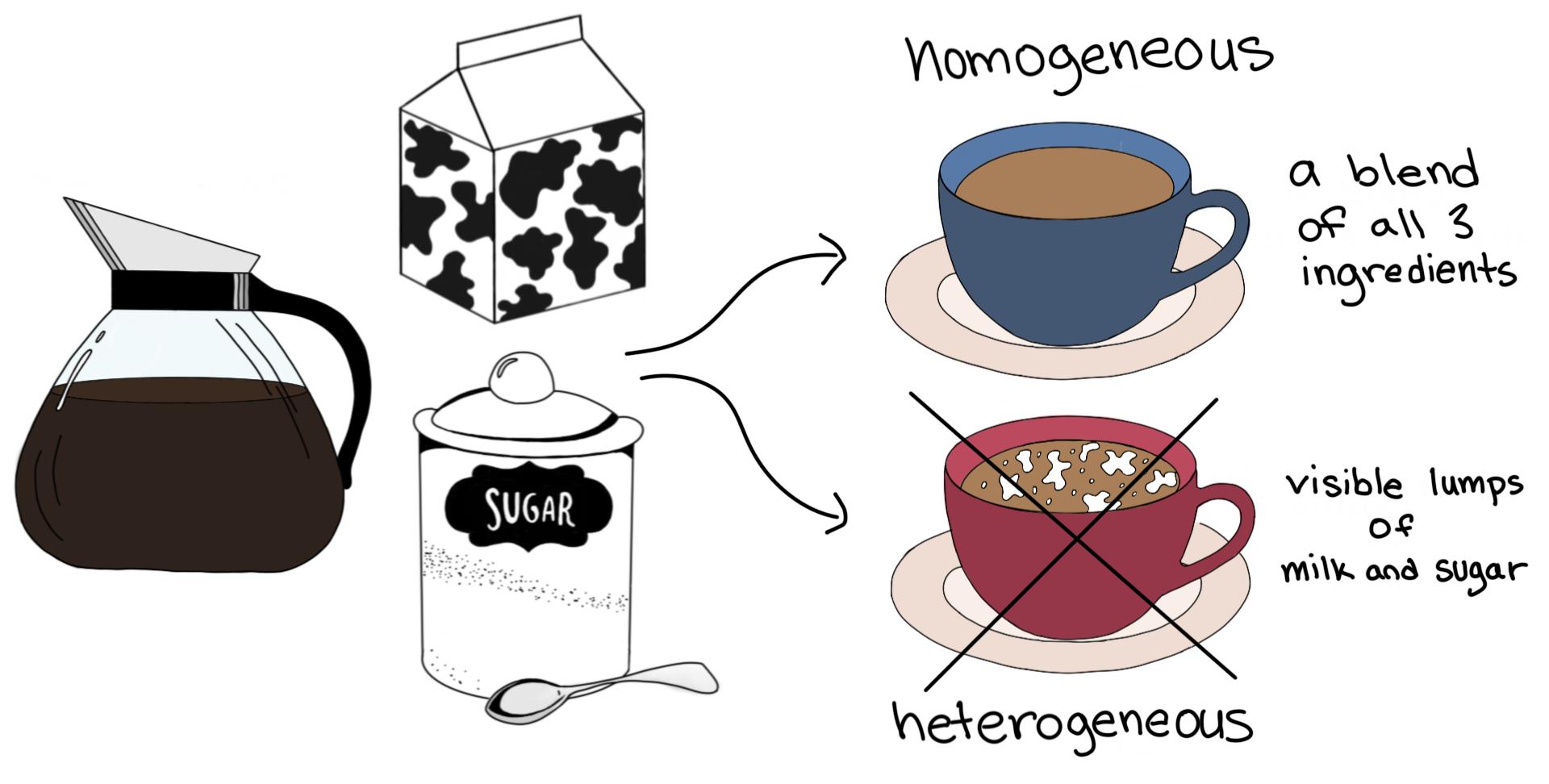



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



3




Homogeneous And Heterogeneous Mixed Populations An Example Of A Download Scientific Diagram




10 Examples Of Mixtures



Mixture




Difference Between Homogeneous And Heterogeneous Reactions Compare The Difference Between Similar Terms




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards
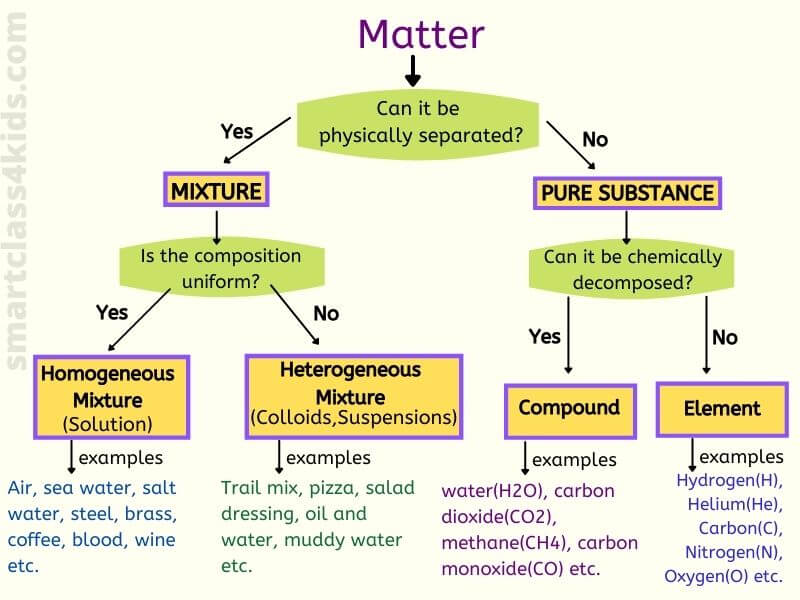



What Is Mixture Homogeneous Mixture Heterogeneous Mixture With Examples




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science
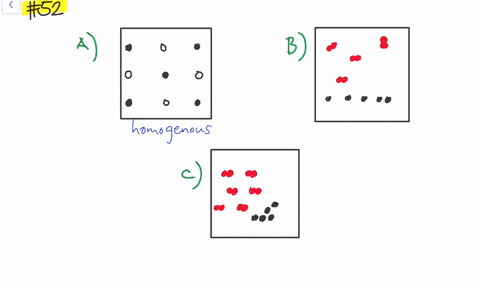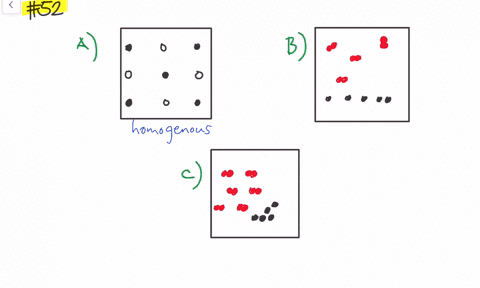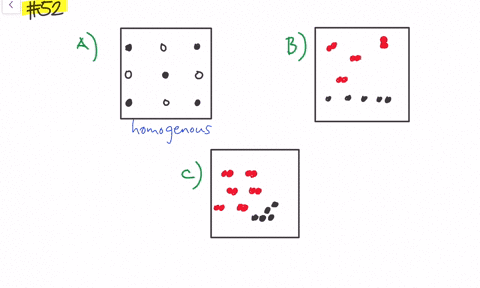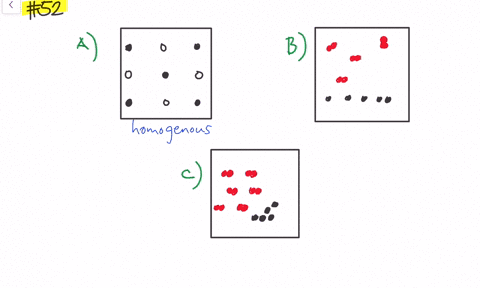



Solved Give Three Examples Each Of Heterogeneous Mixtures And Homogeneous Mixtures




This Presentation Discusses Homogeneous And Heterogeneous Mixtures Provides Examples Explains How A Particle Di Heterogeneous Mixture Pure Products Mixtures




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous Mixture Definition Examples Tutors Com




Homogenous Compounds And Mixtures Homogeneous Mixture Heterogeneous Mixture




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous And Heterogeneous Mixtures Card Sorting Activity By Elly Thorsen




Classes Of Matter Elements Compounds And Mixtures Matter




What Is A Homogeneous Mixture Definition And Examples




A Example Stimulus Sequences From The Heterogeneous And Homogeneous Download Scientific Diagram




Homogeneous And Heterogeneous Mixtures Card By Elly Thorsen Teachers Pay Teachers Heterogeneous Mixture Sorting Activities Persuasive Writing Prompts




Heterogeneous And Homogeneous Mixture Sort Next Generation Science Standards Chemical And Physical Changes Sorting




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool




What Is A Heterogeneous Mixture Definition And Examples




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry




Q2 Differentiate Between Homog Lido




What S The Difference Between Heterogeneous And Homogeneous Mixtures Physical Science Examples Of Mixtures Science Resources



Homogeneous Vs Heterogeneous Matter Worksheet Answers Nidecmege



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



What Are Some Examples Of Homogeneous Mixtures And Heterogeneous Mixtures Enotes Com




Properties Of Homogeneous Mixture The Difference Between Heterogeneous And Homogeneous Mixtures




Heterogeneous And Homogeneous Mixtures With Examples Study Guide Brighthub Education




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Heterogeneous And Homogeneous Mixture Differences Videos Examples




Do Now Which Mixture Is Homogeneous And Which One Is Heterogeneous And Why Quest Tuesday Hand In Mixture Lab Pennies Ppt Download



Homogeneous Heterogeneous Mixture Definition Examples Selftution




What Is A Heterogeneous Mixture In Science Quora



Mixtures Youtube



1




Heterogeneous And Homogeneous Mixtures Project By Miss Let S Do Math
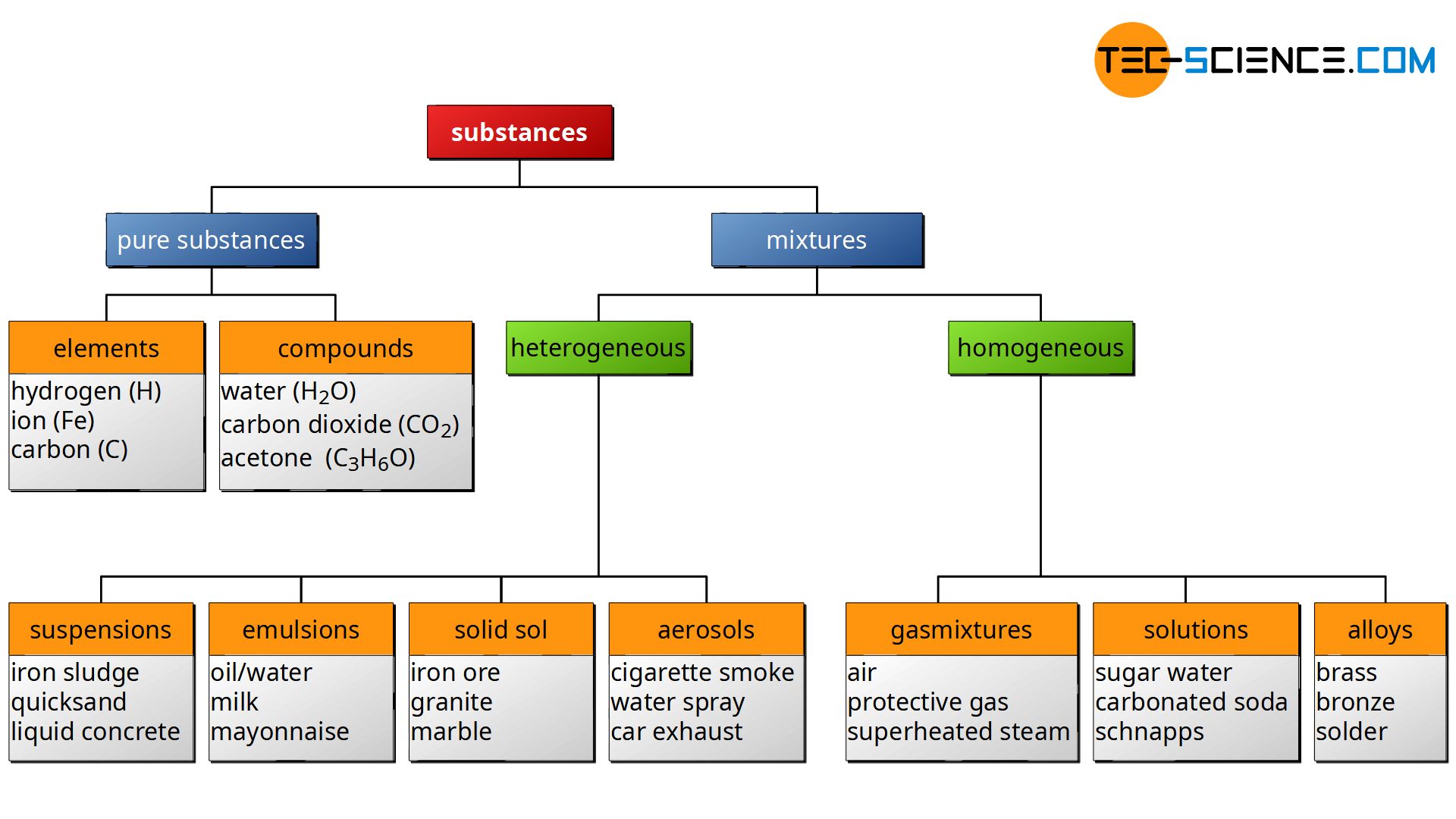



Classification Of Matter Tec Science




Heterogeneous And Homogeneous Mixture Differences Videos Examples
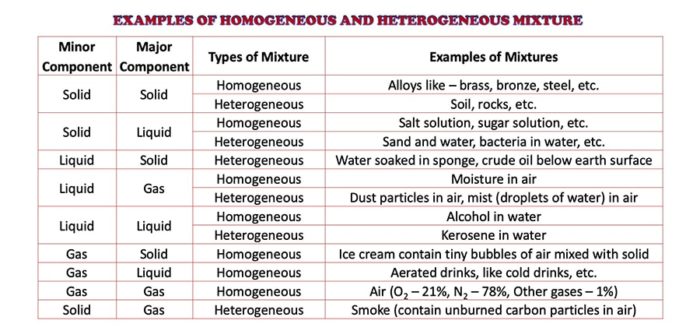



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Pure Substances And Mixtures Unit 2 Matter Element
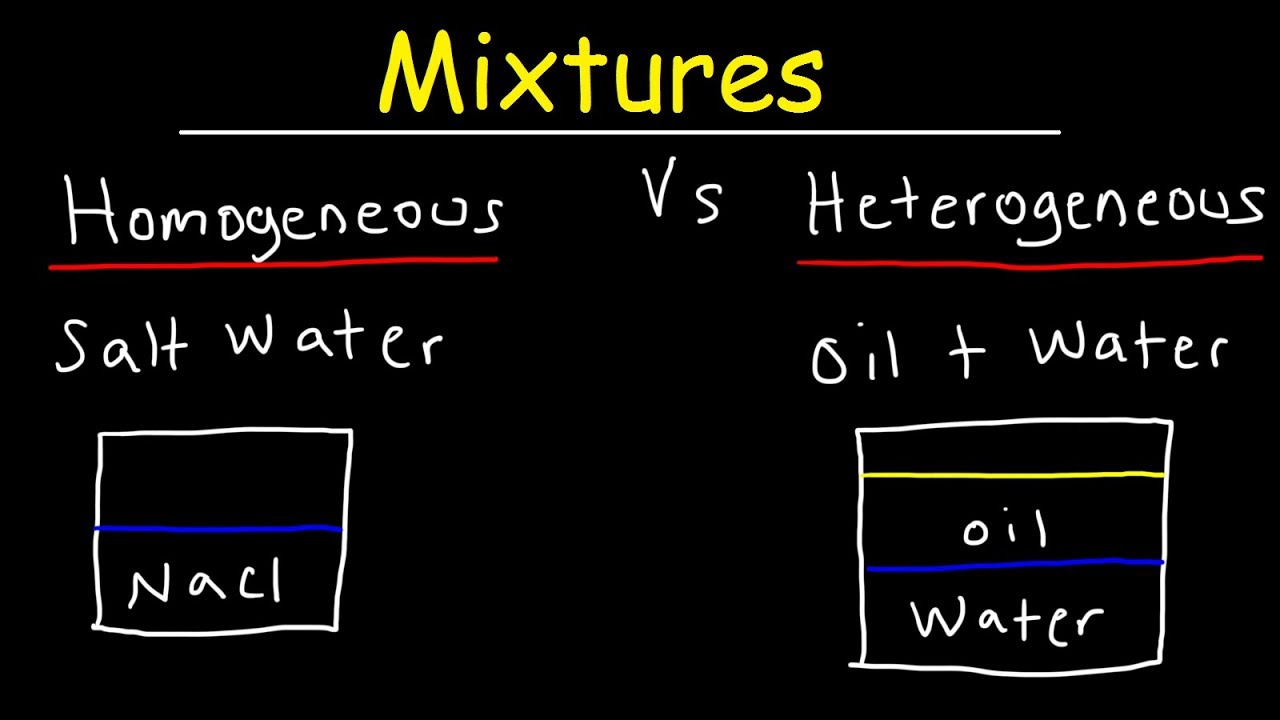



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube



1




Homogeneous And Heterogeneous Mixture Heterogeneous Mixture Homogeneous Mixture Mixtures




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




Homogeneous And Heterogeneous Mixtures Identify The Examples Youtube




Homogeneity And Heterogeneity Wikipedia



Heterogeneous And Homogeneous Mixtures Write And Draw Worksheet By Elly Thorsen
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



10 Heterogeneous And Homogeneous Mixtures




Elements Compounds Homogeneous Mixtures Heterogeneous Mixtures The Following




What Is A Homogeneous Reaction A Reaction Where All Chegg Com




05 Homogeneous And Heterogeneous Mixtures Chemistrysolutions
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples




Chemistry For Kids Chemical Mixtures
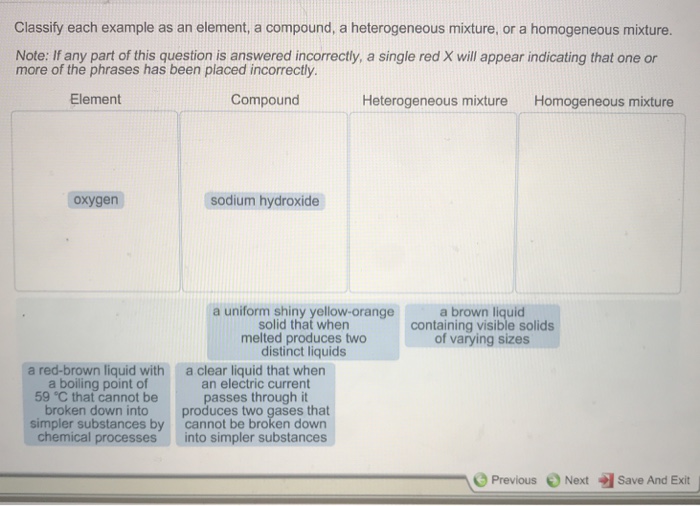



Classify Each Example As An Element A Compound A Chegg Com


